Related Post
18 October, 2022
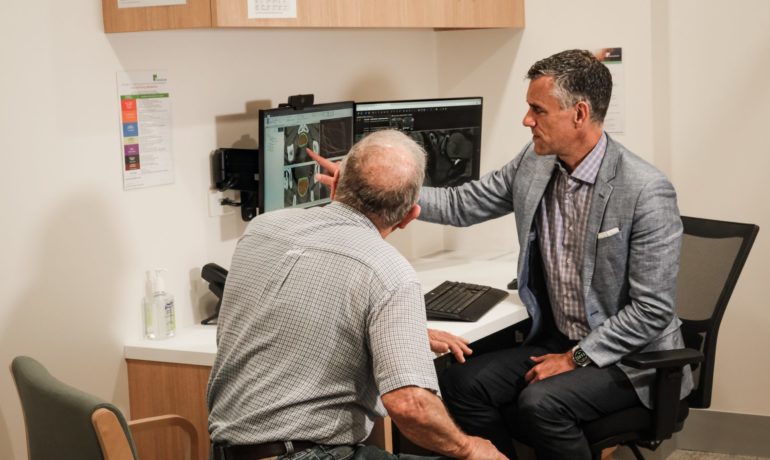
NINJA Radiation Therapy Trial participant tells his journey to treatment
The journey to becoming the 200th participant for the
6 September, 2022
The gift that kept on giving
The NewyWay Coffee Collaboration Newcastle marketing agency, The Marketing