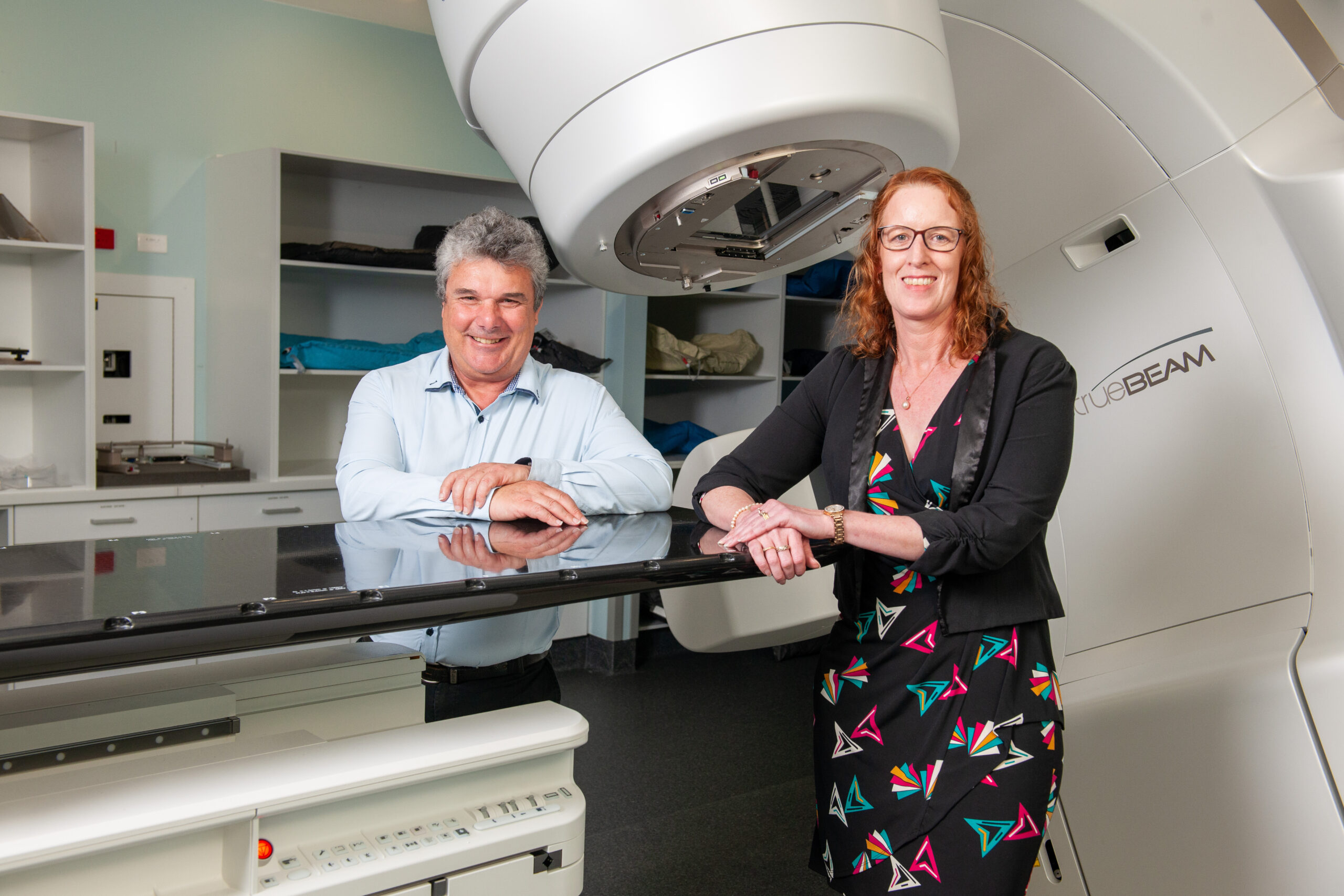
To conduct world-class clinical trials with impactful results, it is vital that data is accurate. Our Radiation Therapy Quality Assurance (RTQA) program ensures every element of the trial data collection process is of the highest quality.
TROG Cancer Research has been conducting multicentre clinical trial QA for more than 30 years.
Quality Assurance (QA) in clinical trials establishes a robust framework to monitor protocol compliance, ensuring data integrity and safeguarding participant safety.
This ensures data is accurate and translatable, and that the trial findings will be published and adopted into clinical practice to improve outcomes.
QA ensures that centres conducting clinical trials have the right experience, equipment, procedures, and knowledge of the protocol to safely and meaningfully participate in the trial.
TROG Cancer Research is committed to collaborating with both national and international experts and continues to review international standards for credentialing new techniques and technologies in radiation oncology.
Technologically advanced software and procedures are continually being incorporated into the TROG Cancer Research QA program. This ensures our researchers have access to the best available resources for conducting their research.
Radiation therapy treatment data
Imaging data (including diagnostic baseline, follow-up, progression imaging)
Interventional oncology data
Nuclear medicine imaging and data
When conducting clinical trials across multiple centres, trial QA promotes;

Participant safety and quality treatment

Trial protocol compliance
Standardisation and harmonisation of treatment, imaging and trial data

Trial quality and integrity
Robust and translational data
The TROG Radiation Therapy QA team, led by Alisha Moore, consists of highly skilled staff members including quality assurance radiation therapists, a quality assurance research officer, and medical physicist. The seven-member team has more than 100 years of combined experience.


A novel quality assurance credentialing program was implemented as part of the TROG 18.06 FIG study, involving credentialing nuclear medicine physicians prior to trial activation. The system was developed to ensure consistency and accuracy in FET-PET lesion delineation for glioblastoma patients.
Data was evaluated by expert reviewers to assess if the FET-PET lesion delineation met protocol standards. Benchmarking results were collated and analysed with data from 10 centres across Australia evaluated. The novel credentialing program was shown to increase expertise across the study sites, and improve standardisation for trial patients.
The TROG RTQA team worked closely with the FASTRACK II study team to support analysis of the dose-effect relationship of a novel form of radiation therapy called stereotactic ablative body radiotherapy (SABR) in patients with kidney cancer. The TROG 15.03/ANZUP 16001 – FASTRACK II trial measured patients’ loss of renal function after treatment as a secondary endpoint. The RTQA program facilitated the collection and transfer of imaging data for subsequent analysis.
Renal function image sets were rigidly registered to the planning CT, where kidneys were segmented to calculate dose-response curves. At 12 and 24 months post-treatment, the study quantified, for the first time in a multicentre setting, the dose–effect relationship of SABR on renal function. The study reported that the major loss of renal function occurred in regions of high radiation dose, where the dose-response curve converged to a plateau (i.e. additional radiation dose cause little further decline beyond this point). This data is valuable as it helps radiation oncologists understand the dose-effect of RT to the kidneys which can potentially help improve the quality of life for future patients.
Contact us
If you require any further information or want to discuss collaborating with TROG, don’t hesitate to contact us at: qa@trog.com.au
Have your say – provide feedback today
The TROG QA team are always seeking to improve processes and systems. We value positive and constructive feedback from our members and clinical trials colleagues.
If you have any feedback or suggestions relevant to TROG QA, please feel free to complete our feedback survey or contact us.