A cancer clinical trial is a research study that investigates new tests or treatments for patients who have been diagnosed with cancer. It involves the testing of new treatments or finding ways to improve existing treatments but may also involve tests and interventions to prevent and detect cancer early.
Cancer clinical trials help us find out if a promising new cancer treatment is effective and offers more benefits than current standard treatment options. Significant advances occur thanks to hundreds of thousands of patients entering clinical trials across the world.
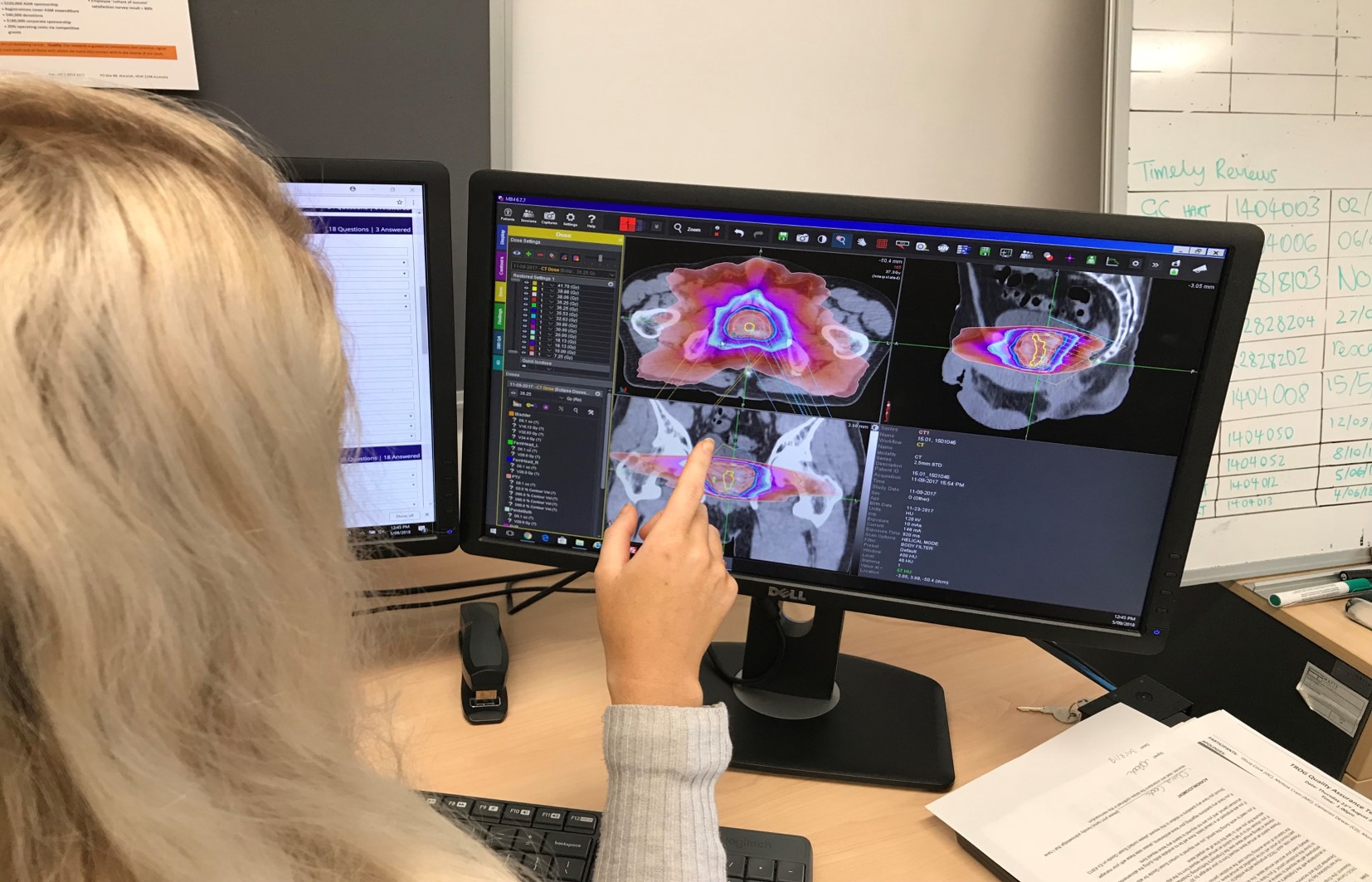
Through our clinical trials, TROG Cancer Research has been able to aid in the advancement of cancer treatment and management.
TROG Cancer Research clinical trials are professionally researched and supported and are at the forefront of expert opinion on how to improve the outcomes of people living with cancer.
A TROG Cancer Research clinical trial needs the approval of TROG members and other experts in related fields; hospitals and cancer centres; human research ethics committees; government funding bodies; and, most importantly, patients. To provide an informed consumer review, TROG Cancer Research clinical trials involve a consumer as part of the Trial Management Committee.
Before you participate in a trial, the researchers will make sure you understand all the possible risks, benefits, and alternatives to the study and you are encouraged to ask any questions about the clinical trial. All clinical trials are regularly monitored to ensure the rights, safety, and wellbeing of participants, as well as the integrity of the data, is protected at every stage of the trial.



Cancer clinical trials can involve people of all ages, from children to older adults. Taking part is completely voluntary and participants can withdraw at any time with no negative impact on the quality of their medical treatment or relationship with their doctor.
Some cancer clinical trials need healthy participants to test the safety of new interventions or tests, particularly in early stage trials. In these trials, healthy volunteers can be compared with cancer patients. They receive the same test, procedure, or drug that the patient group receives, and researchers can compare the effects and side effects of the new test, procedure, or drug between the two groups.
Many cancer clinical trials need participants who have the specific disease that the new intervention targets. TROG Cancer Research outline the participant requirements for each trial in a Clinical Investigation Plan and in a detailed Participant Information. This helps the patient and researcher to determine whether the patient is best suited for the clinical trial.